You are here:
- Home
- Piperidines
- 1-Piperidinepropanol
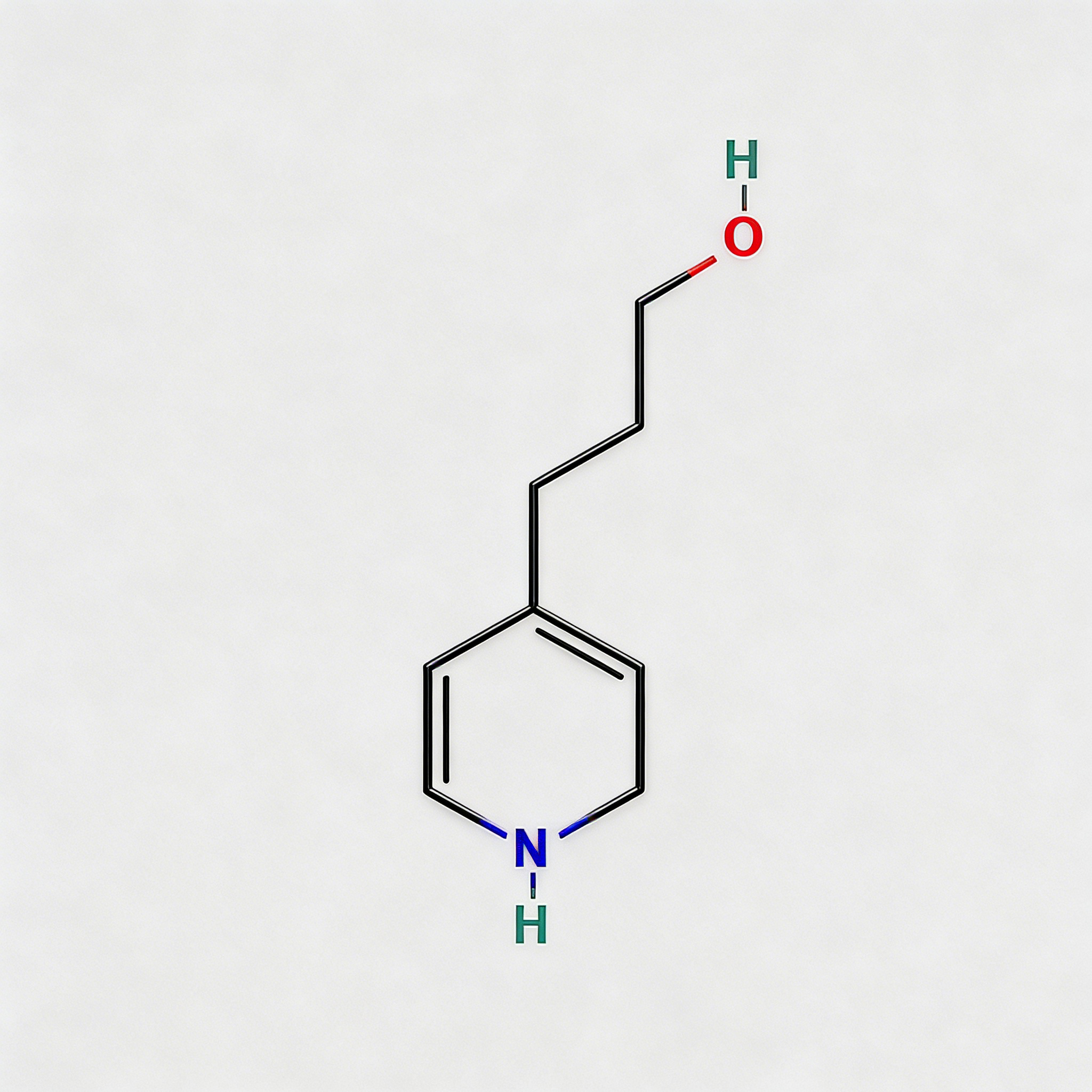
1-Piperidinepropanol
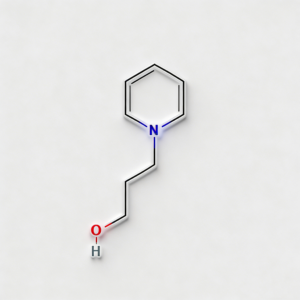
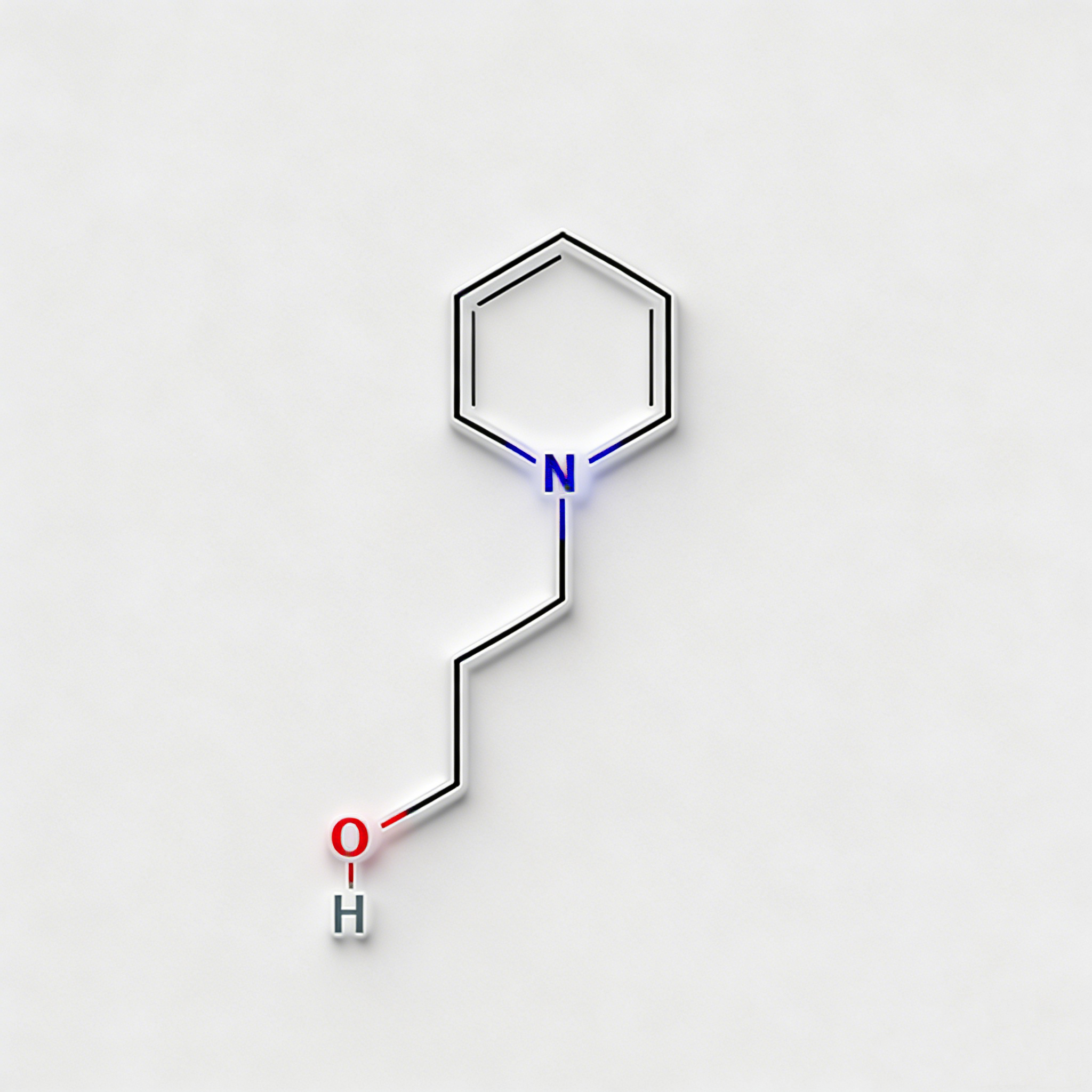
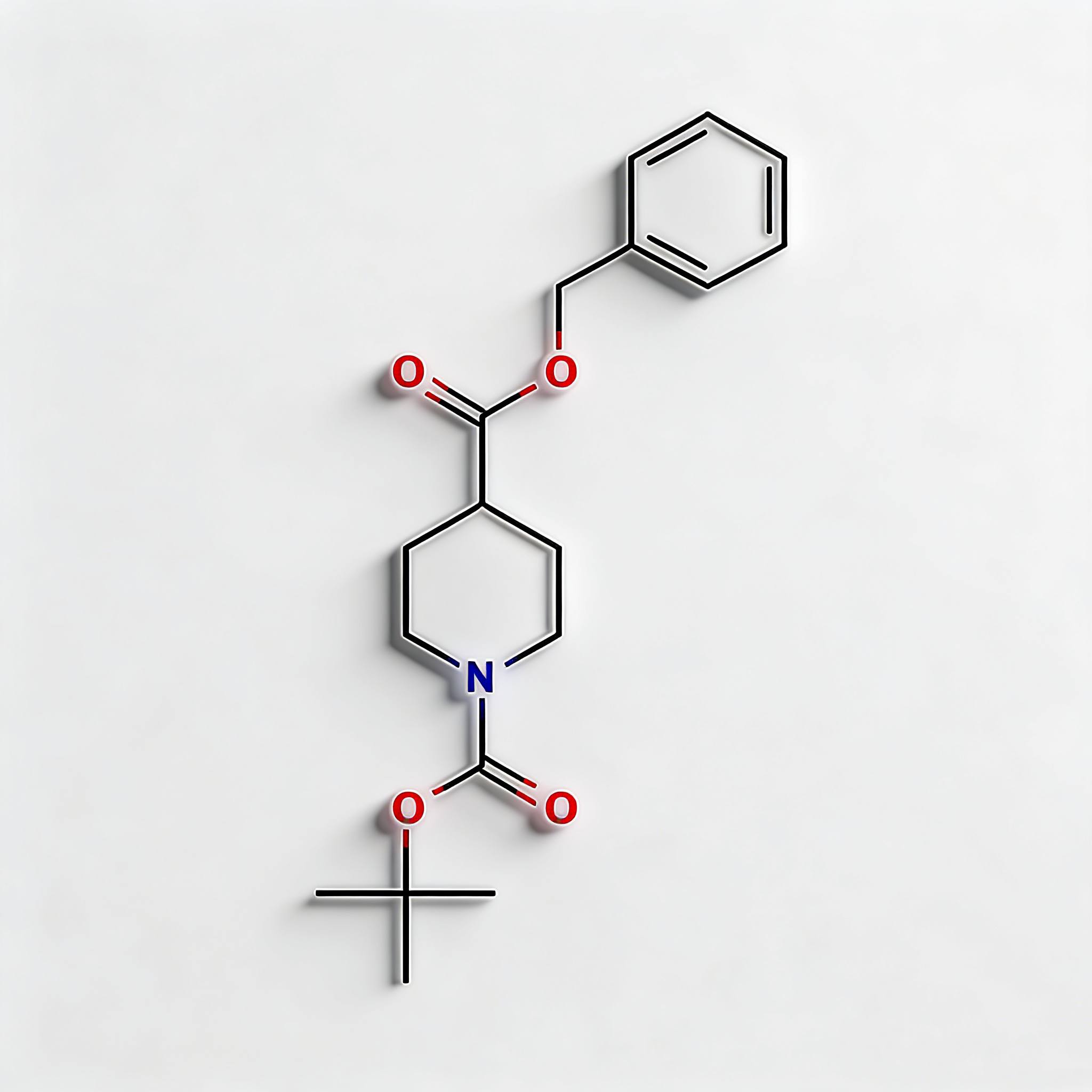
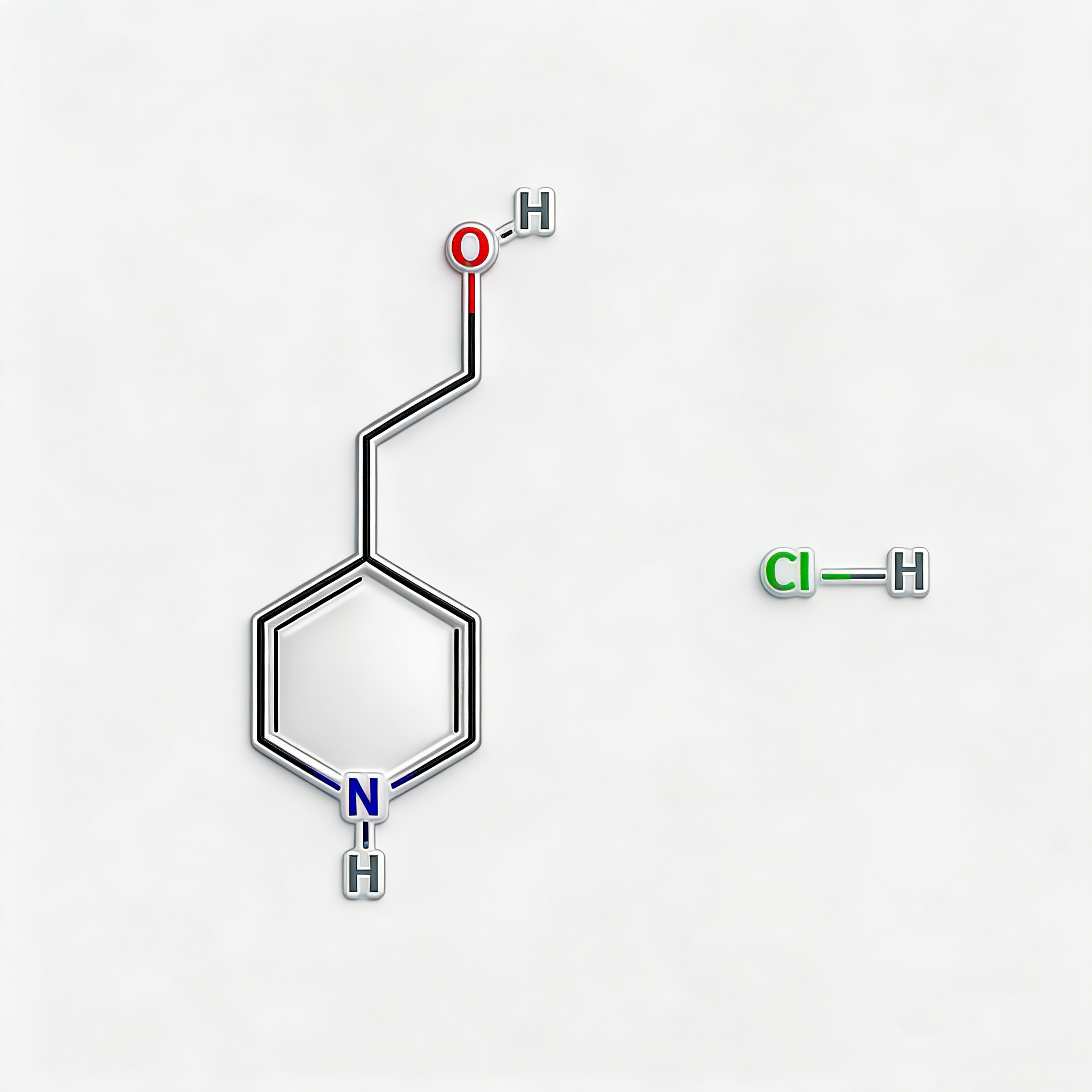
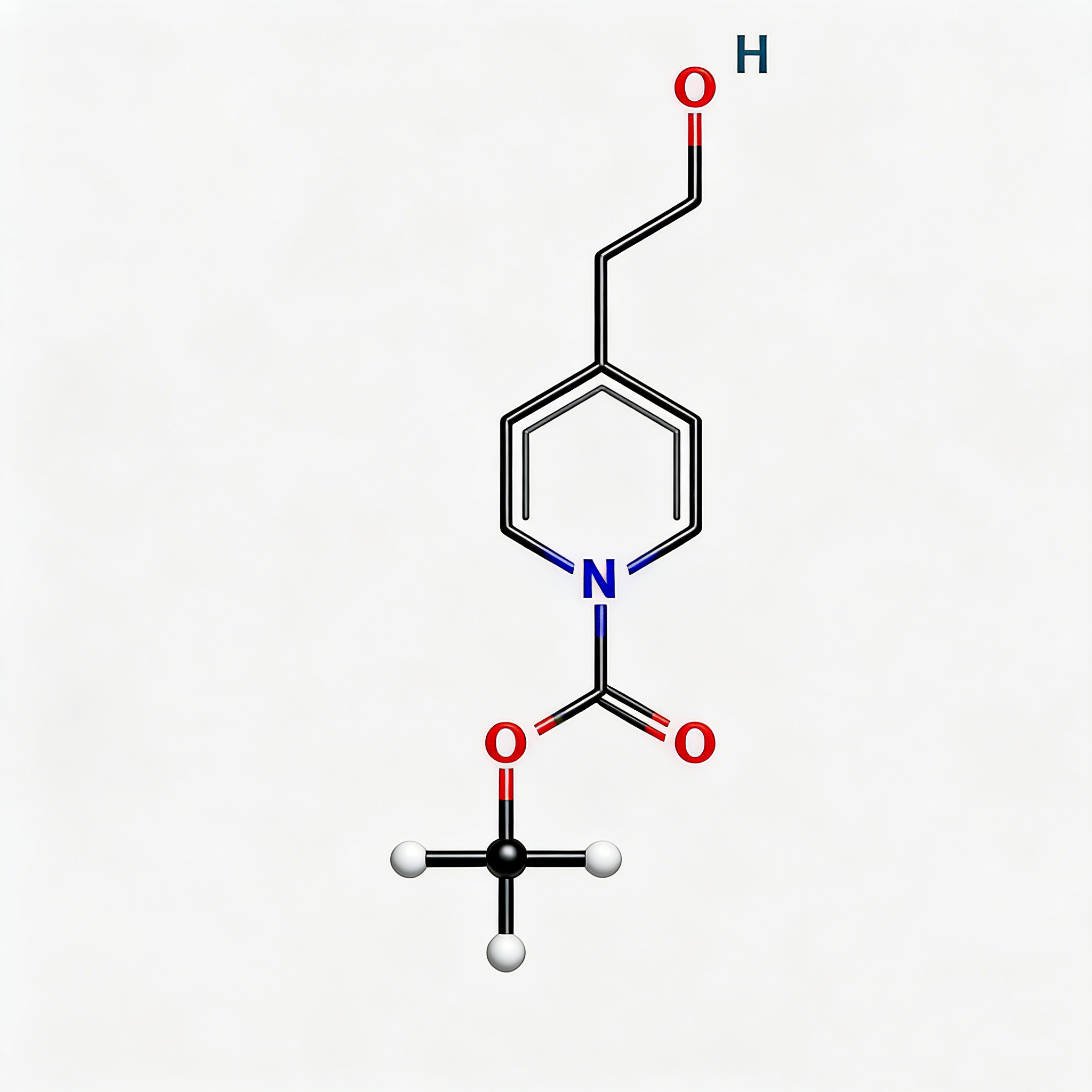
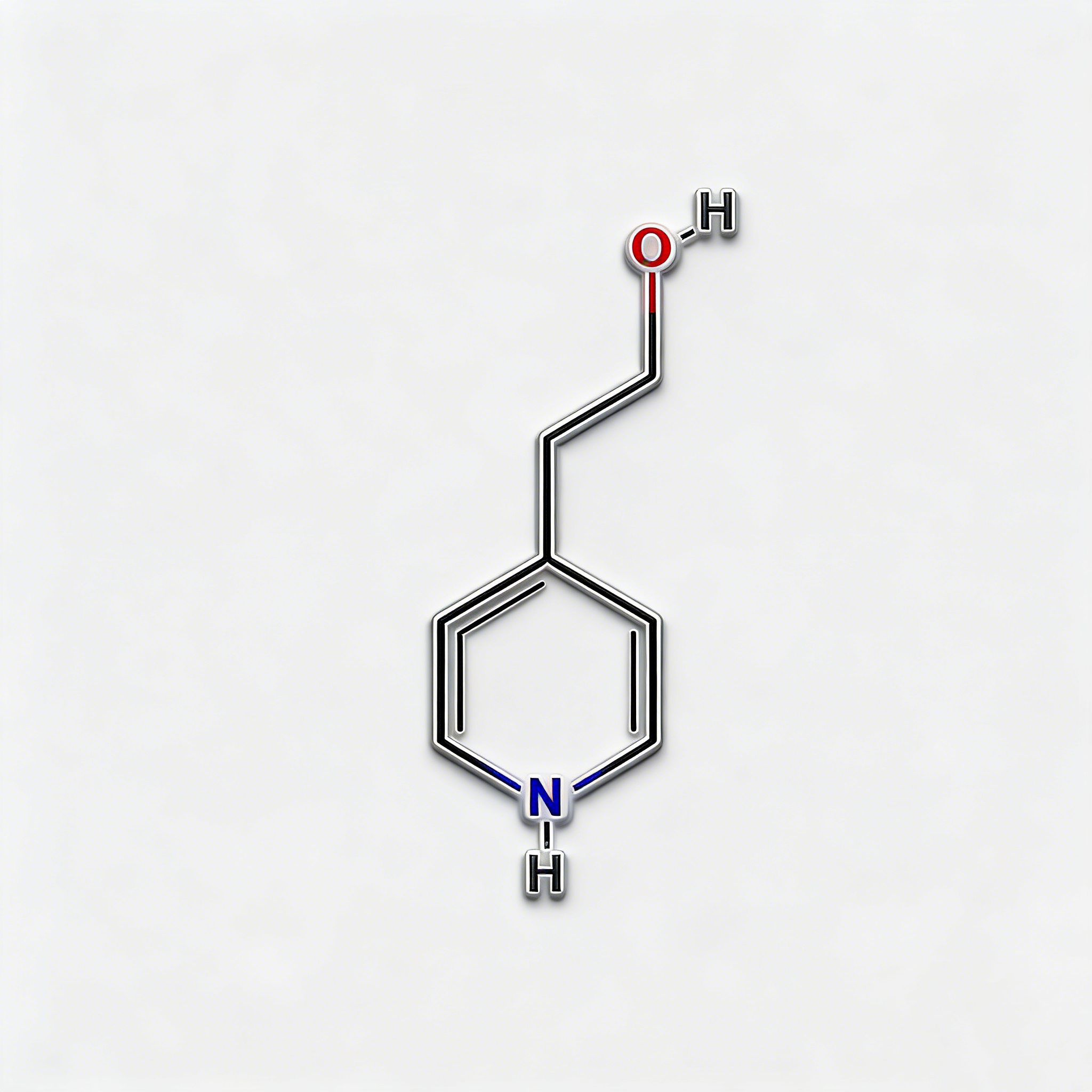
1-Piperidinepropanol (CAS 104-58-5), also known as 3-(1-piperidinyl)-1-propanol, is a strategically functionalized N-substituted piperidine derivative engineered for pharmaceutical intermediate synthesis, ligand development, and polymer additive applications. This molecule integrates a saturated piperidine core—a six-membered nitrogen-containing heterocycle prevalent in bioactive molecules—with a 3-hydroxypropyl side chain attached directly to the nitrogen atom (N1-position). The tertiary amine nitrogen provides basicity and nucleophilicity for salt formation or quaternization, while the primary alcohol terminus enables esterification, etherification, oxidation to aldehyde/acid, or conversion to halides for further functionalization. Compared to C-substituted piperidines (e.g., CAS 622-26-4), the N-substitution pattern offers distinct electronic properties, enhanced metabolic stability at the amine center, and reduced steric hindrance around the ring carbons. This intermediate is particularly valuable for constructing CNS-active pharmaceuticals, antihistamines, muscle relaxants, and polymer stabilizers where the piperidinyl-propanol motif balances lipophilicity with hydrogen-bonding capability. Manufactured under controlled conditions, it supports drug discovery programs requiring modular access to tertiary amino-alcohol architectures with orthogonal derivatization pathways.
Property
|
Product Name
|
1-Piperidinepropanol
|
|
CAS Number
|
104-58-5
|
|
Chinese Name
|
1-哌啶丙醇
|
|
Synonyms
|
3-(1-Piperidinyl)-1-propanol; 1-(3-Hydroxypropyl)piperidine; N-(3-Hydroxypropyl)piperidine; C₈H₁₇NO heterocycle
|
|
Chinese Synonyms
|
3-(1-哌啶基)-1-丙醇; 1-(3-羟丙基) 哌啶; N-(3-羟丙基) 哌啶; 哌啶丙醇
|
|
Molecular Formula
|
C₈H₁₇NO
|
|
Molecular Weight
|
143.23 g/mol
|
|
Purity
|
≥ 98% (GC/HPLC area normalization)
|
|
Product Category
|
Pharmaceutical Intermediates / Piperidine Derivatives / Tertiary Amino Alcohols
|
|
Appearance
|
Colorless to Pale Yellow Liquid
|
|
Boiling Point
|
~250-260°C at 760 mmHg (estimated)
|
|
Density
|
~0.99 g/cm³ at 20°C (estimated)
|
|
pKa
|
~10.0-10.5 (tertiary amine, estimated)
|
|
Storage Conditions
|
Store in a cool, dry, well-ventilated area away from light and moisture. Keep container tightly closed under inert atmosphere (nitrogen). Protect from strong oxidizing agents to avoid amine oxidation (N-oxide) or alcohol degradation.
|
|
Shelf Life
|
24 months under recommended storage conditions
|
|
Packaging
|
100mg/vial, 500mg/vial, 1g/bottle, 5g/bottle, or customized according to customer requirements
|
|
Applications
|
CNS drug synthesis (antihistamines/muscle relaxants), tertiary amine ligand development, polymer additive precursor (UV stabilizers), alcohol derivatization (esterification/etherification/oxidation), quaternary ammonium salt synthesis
|
|
Reactivity
|
Suitable for alcohol esterification/etherification, oxidation to aldehyde/acid, conversion to halides, amine salt formation, quaternization, N-oxide formation, and heterocyclic cyclization
|
|
Special Note
|
✅ N-Substituted Tertiary Amine—distinct from C-substituted secondary amines (CAS 622-26-4). Dual reactivity (tertiary amine + primary alcohol). Part of our piperidine building block series.
|